About Chlorite
From a Greek word meaning green, in allusion to the common color.
Chlorite hand-specimen
Formula: (Mg,Fe)3(Si,Al)4O10(OH)2 • (Mg,Fe)3(OH)6
System: Monoclinic
Color: Green, yellowish green, olive green, blackish green, bluish green, white, pink
Lustre: Greasy, Pearly, Dull
Hardness: 2–2½
Density: 2.6–3.3
System: Monoclinic
Color: Green, yellowish green, olive green, blackish green, bluish green, white, pink
Lustre: Greasy, Pearly, Dull
Hardness: 2–2½
Density: 2.6–3.3
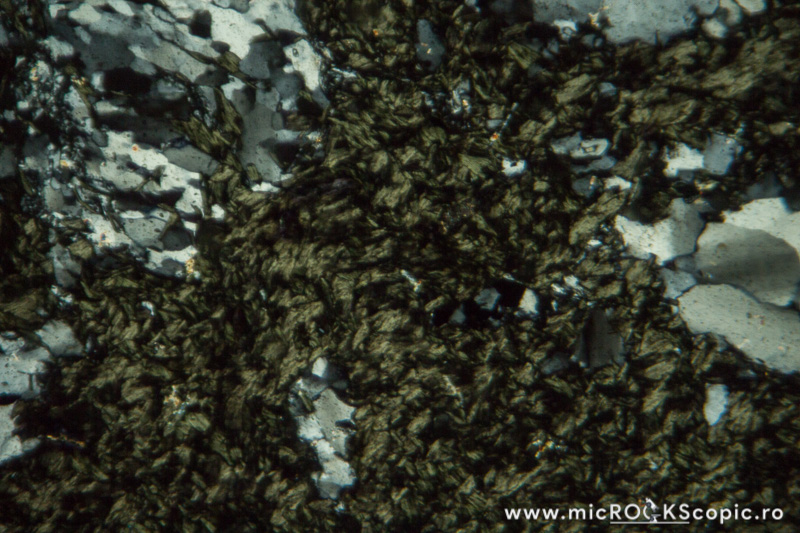
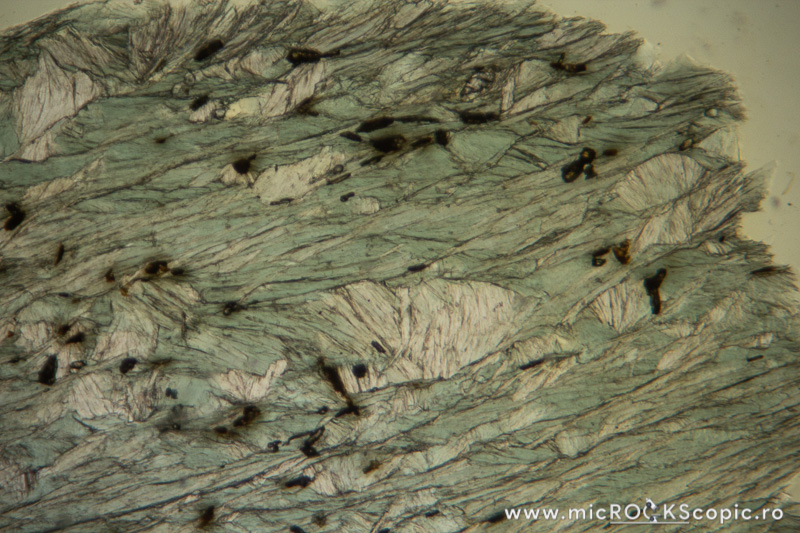
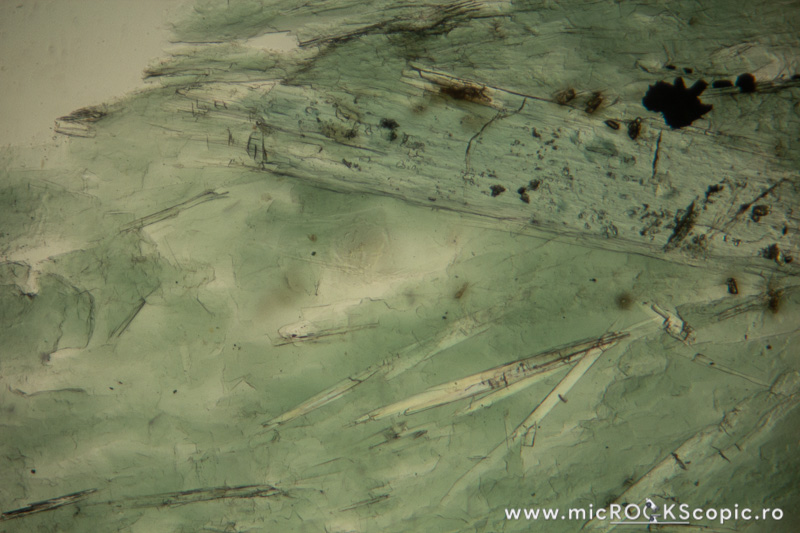
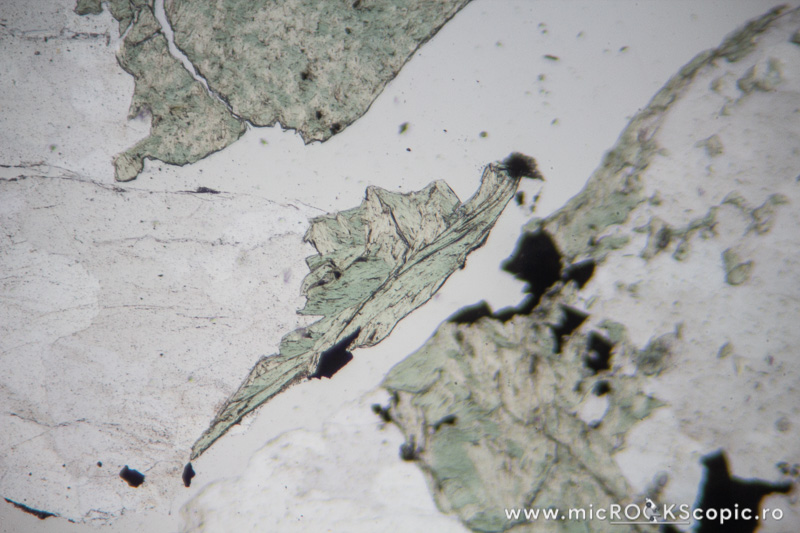
Chlorite PPL properties
Relief: Low-Moderate positive
Habit/Form: Rare crystals are tabular parallel to (001) with a roughly hexagonal outline. Commonly found as plates or scales similar to the micas. In sediments, chlorite is a common constituent of the clay fraction and may form oolitic or spherulitic balls similar to glauconite.
Color: Colorless to pale green and greenish or green
Pleochroism: Moderate
Cleavage: Perfect in one direction {001}; in basal sections (z-axis) has no cleavage.
Habit/Form: Rare crystals are tabular parallel to (001) with a roughly hexagonal outline. Commonly found as plates or scales similar to the micas. In sediments, chlorite is a common constituent of the clay fraction and may form oolitic or spherulitic balls similar to glauconite.
Color: Colorless to pale green and greenish or green
Pleochroism: Moderate
Cleavage: Perfect in one direction {001}; in basal sections (z-axis) has no cleavage.
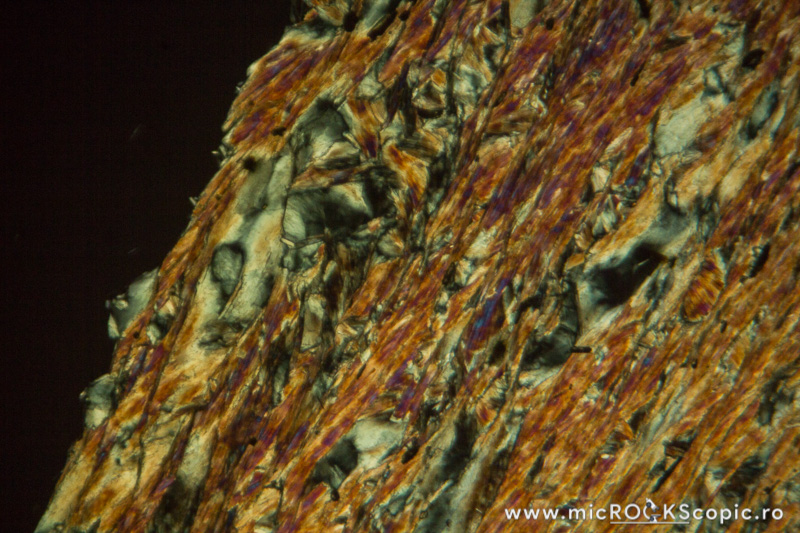
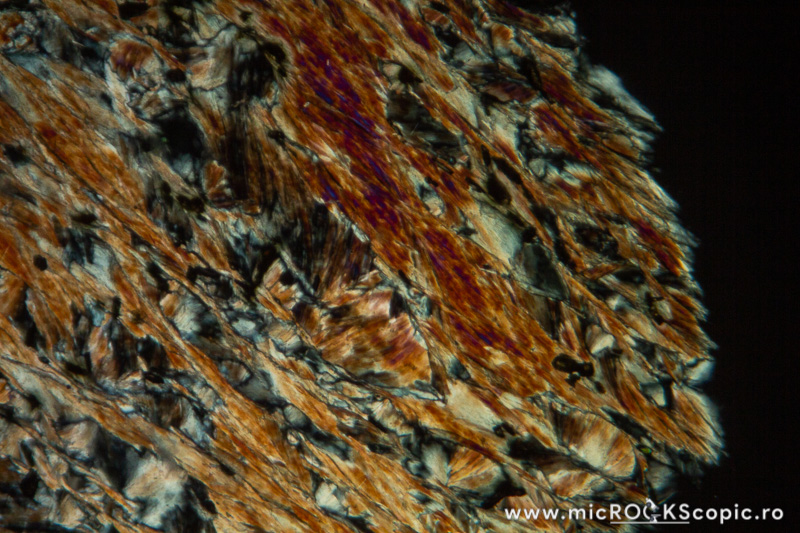
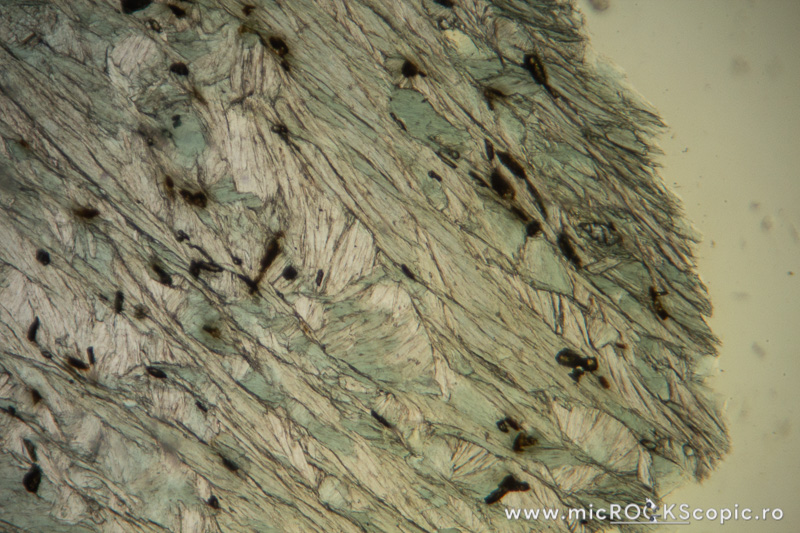
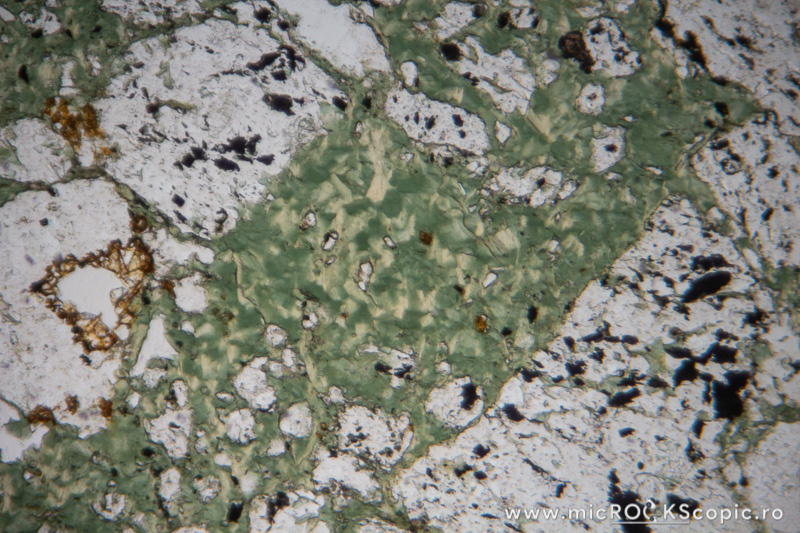
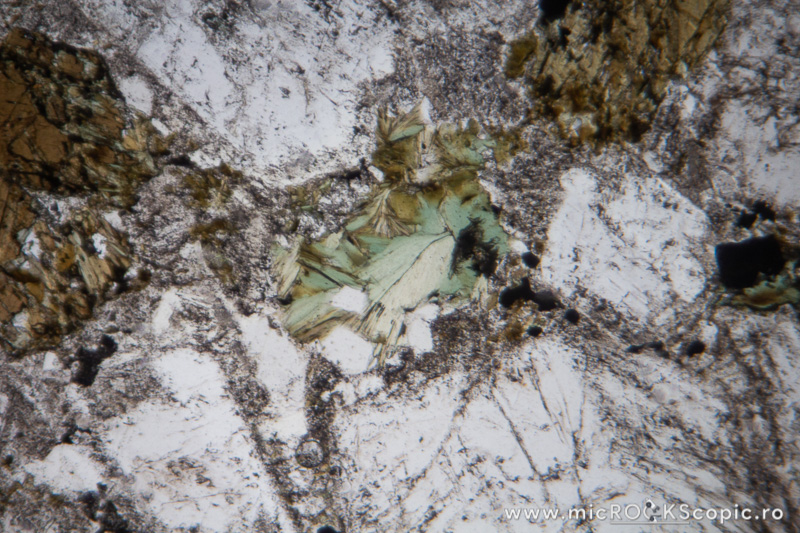
Chlorite XPL properties
Isotropy/Anisotropy: Anisotropic
Interference color: Order I – II (often ultrablue interference color)
Extinction angle: 0 – 10°
Twins: Multiple twinning on {001} is common
Uniaxial/Biaxial: Biaxial (+) (-)
Optic axial angle (2V): 2V 0 – 40°(-)
2V 0 – 60°(+)
Interference color: Order I – II (often ultrablue interference color)
Extinction angle: 0 – 10°
Twins: Multiple twinning on {001} is common
Uniaxial/Biaxial: Biaxial (+) (-)
Optic axial angle (2V): 2V 0 – 40°(-)
2V 0 – 60°(+)
Chlorite distinguishing features under the microscope
Get Geology Toolkit Premium for more features of Chlorite thin section under the microscope.
References
- Deer, W. A., Howie, R. A., & Zussman, J. (2013). An introduction to the rock-forming minerals (pp. 498). Mineralogical Society of Great Britain and Ireland, London.
- mindat.org – The Mineral Database
















![[thumb]](http://microckscopic.ro/wp-content/uploads/chlorite_5_05-150x150.jpg)
![[thumb]](http://microckscopic.ro/wp-content/uploads/chlorite_1_15-150x150.jpg)
![[thumb]](http://microckscopic.ro/wp-content/uploads/chlorite_06-1-150x150.jpg)
![[thumb]](http://microckscopic.ro/wp-content/uploads/chlorite_12-1-150x150.jpg)
![[thumb]](http://microckscopic.ro/wp-content/uploads/chlorite_2_04-150x150.jpg)
![[thumb]](http://microckscopic.ro/wp-content/uploads/chlorite_2_09-1-150x150.jpg)
![[thumb]](http://microckscopic.ro/wp-content/uploads/chlorite_3_01-1-150x150.jpg)
![[thumb]](http://microckscopic.ro/wp-content/uploads/chlorite_3_05-1-150x150.jpg)
![[thumb]](http://microckscopic.ro/wp-content/uploads/chlorite_4_01-1-150x150.jpg)
![[thumb]](http://microckscopic.ro/wp-content/uploads/chlorite_4_05-150x150.jpg)
![[thumb]](http://microckscopic.ro/wp-content/uploads/chlorite_6_05-1-150x150.jpg)
![[thumb]](http://microckscopic.ro/wp-content/uploads/chlorite_6_01-1-150x150.jpg)






